Clinical Trial Cohort Management
Streamlining slot allocation operations between clinical research sites (Sites) and biopharmaceutical companies (Sponsors)
Setting the Scene
Managed Enrollment & Precision Medicine
Onestudyteam (OST) offers cloud-based software solutions to both clinical research sites (Sites), research centers and global biopharmaceutical companies (Sponsors) to accelerate clinical trial enrollment. The company's mission is to remove clinical trial operations as the bottleneck on delivering new therapies to patients. The Slot Allocation (also known as managed enrollment) initiative was a strategic attempt by OST to expand their product offering in order to support early phase clinical research studies. We aimed to create a communication tool between Sites and Sponsors.
Early phase trials (I or II), such as dose escalation studies, often have limited enrollment slots that sites compete to fill. Sites request a slot in conjunction with patient groups (i.e. cohorts), or in dose escalation trials, sites request a slot from the sponsor at a specific dose.
Sponsors receive the slot request from the site and decide how to approve and allocate the slot depending on the submission date (first-come-first serve), the cohort in need of more subjects, the closest expected enrollment date for a patient, and whether the site has yet to receive a slot.
The slot approval then begins an accelerated enrollment process where the site must obtain consent, screen the patient, and get final confirmation from the sponsor before officially enrolling the patient into the trial. Slots are not only a tool to enroll patients into the trial as quickly as possible, but also a communication workflow.
Why are we here?
Understanding the Problem
Some clinical trials, such as early phase studies (I or II), have smaller allowed spaces for enrollment and require slotting management tools to track recruitment and patient allocation. These trials often emphasize speedy enrollment because interested patients have exhausted other avenues of treatment. Combined with a limited number of slots, these types of clinical trials are highly competitive.
Despite the need for faster enrollment, traditional slotting workflows are often tedious and time-consuming for both sites and sponsors. Existing workflows rely on several disparate tools and manual communication, which can delay patient allocation and study enrollment. Due to the emphasis on speedy enrollment, it's important to see Slots as not only a communication workflow but also a tool to schedule patients into the trial as quickly as possible.

Excess of tools
Sponsors and sites use email, google forms (or similar forms), phone calls, and multiple spreadsheets to manage and communicate all of this information. The process and tools by which slots are allocated can vary by trial, sponsor, etc, which makes tracking and maintaining updated trial information difficult.No central monitoring location
Sponsor and site teams do not have a centralized way to clearly understand the volume of slot requests across sites, the progress for trial enrollment goals, the associated patient information and approval workflow, and the slot audit history. When the sponsor approves a slot, the site generally receives a timeframe to screen and enroll the patient and can struggle to act quickly, which puts the site at risk of losing that slot allocation.Highly manual process
Using email and phone calls to submit slot requests are time consuming and don't automatically place patients in order of the request submission. Submitting through forms and maintaining spreadsheets can lead to more errors, increased staff time, and unnecessary back and forth between sponsors and sites to ensure both have the necessary information and pertinent updates. It's critical that only eligible patients are enrolled in a study and only when an assigned slot is available and approved by the sponsor.Personas
Identifying our Users
The primary users for this project are employees at biopharma companies across two primary job functions. When considering individual user needs based on role, we can break it down a little further.

“I need information at a glance so that I can easily identify successes and problem areas. It is important for me to have the ability to drill down into problem areas so that I can try to fix them.”
Trial Manager
Bio
Sponsor manager is responsible for the oversight of recruitment and enrollment across an entire trial at the global level. This person may be our primary point of contact, and is likely reviewing the Enrollment Report/StudyTeam data on a weekly basis (if not more frequently). If protocol amendments are needed, this person is likely the decision maker. The persona responsible for the overall success of the trial.
Education
Bachelor or Masters degree in a Life Science
(biology, microbiology, pharmacology, molecular biology, toxicology or immunology)
Traits
- Excellent computer skills
- Excellent communication and organizational skills
- Knowledge of good clinical practice (GCP)
Goals
- See overall recruitment status for my region (behind, on-target, ahead)
- Identify high incidents of failure for criteria so that I can modify protocol if needed
- See contact information for necessary Sponsor staff when I have questions
- Identify successful/active and unsuccessful/inactive sites during enrollment
- Identify successful and unsuccessful recruitment vendors

Photo by Christina @ wocintechchat.com on Unsplash
“Research gives me the opportunity to make a difference in patients' lives. I feel that I play a critical role in developing new treatments and therapies that can improve the health and well-being of patients around the world”
Clinical Research Coordinator (CRC)
Bio
Liz is a Clinical Research Coordinator. She has been working in the field of clinical research for over 10 years, starting as a research assistant before becoming a CRC.
Education
Bachelor of Science in a relevant subject, such as clinical research administration or health sciences, public health, or microbiology
Traits
She has excellent communication and interpersonal skills, which allow her to maintain strong relationships with study participants, investigators, and sponsors.
- Detail-oriented add highly organized
- Responsible professional who thrives in high-pressure environments
- High level of integrity
Responsibilities
As a Clinical Research Coordinator, Liz is responsible for the overall management and coordination of clinical trials. Her responsibilities include:
- Developing and implementing study protocols in collaboration with investigators
- Ensuring compliance with regulatory requirements, such as informed consent, data management, and safety monitoring
- Screening and enrolling study participants, while maintaining their confidentiality
- Collaborating with investigators and sponsors to ensure the smooth operation of the trial
- Ensuring the protection of the rights and welfare of study participants
Discovery & Research
Mapping our User's Journey
We identified a number of actively enrolling early phase trials being conducted by our existing clients. After identifying our potential users, we conducted a series of interviews to better understand their current workflow. Our research highlighted an interesting problem, each Sponsor had their own unique process to manage their early phase enrollment. After synthesizing the data gathered, we attempted to identify similarities in the differing workflows.
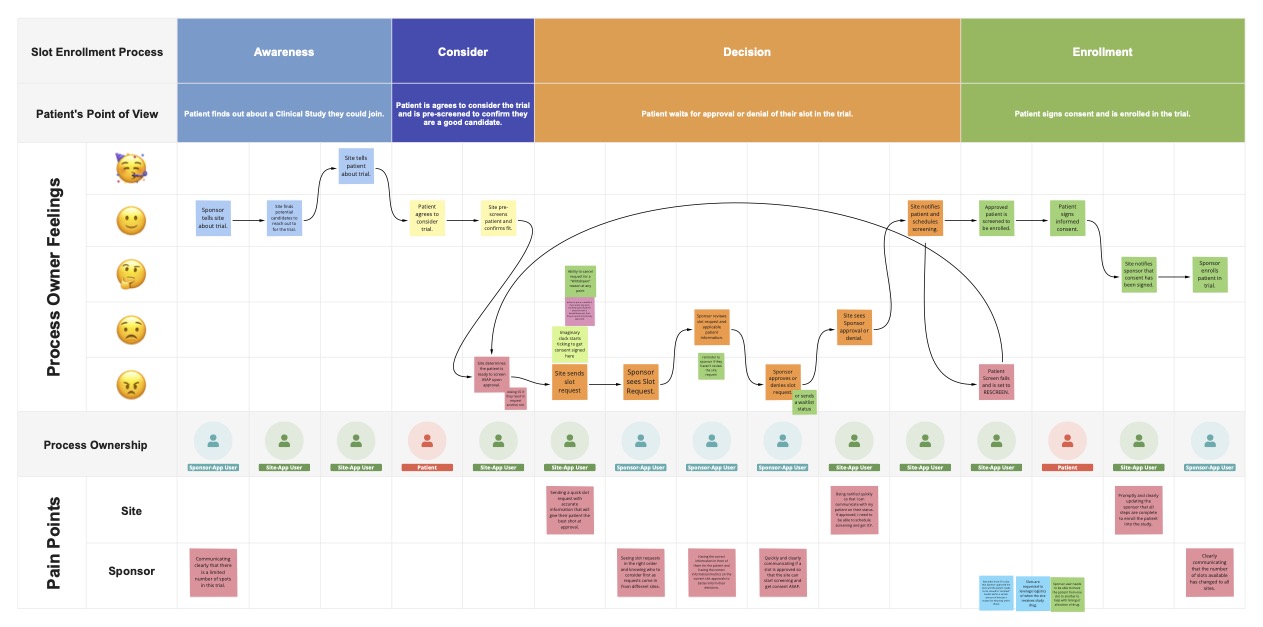
Analyzing what we learned
After conducting several interviews with Sponsor users, we were able to pinpoint the need for the following core capabilities:
- Access a central location to manage slot requests across sites
- Review and respond to slotting requests from sites
- Identify new and updated requests that require immediate review
- Receive automated email notifications for new slot updates
- Monitor enrollment progress for slots
- Design and implement abandon cart email
- Keep track of the history of slot status updates
- Configure slotting information needed from sites
Defining Features Goals
Beginning the Design Process
The StudyTeam Slot Management solution streamlines manual processes by providing a unified system to keep track of eligible candidates and to access real-time slotting updates from sponsors. It is a communication workflow that allows sites to send slot requests and sponsors to review and manage slot updates to more efficiently get patients into trial slots.
Improve Communication
Automate the communication processes between Sites and Sponsors
Provide an Audit Trail
Capture a complete history of patient allocation for the duration of the trial
Centralize Reporting
Track and visualize data in centralized location
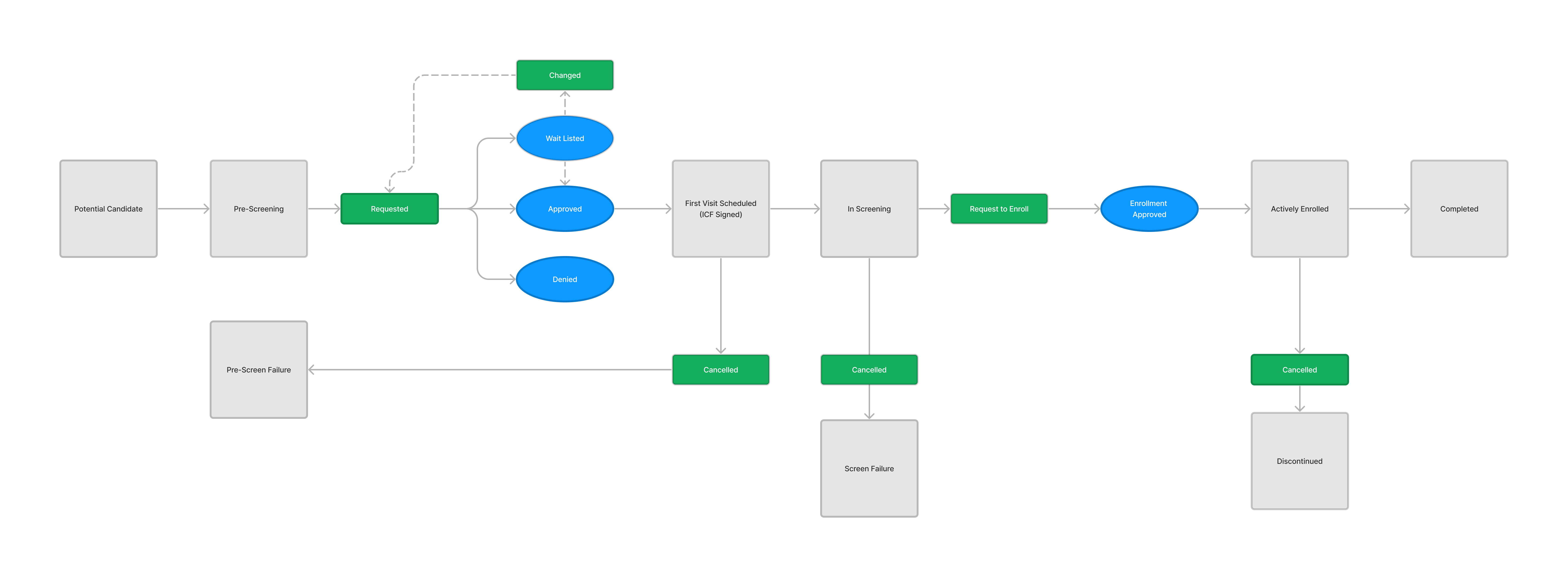
Jammin' in Lo-fi
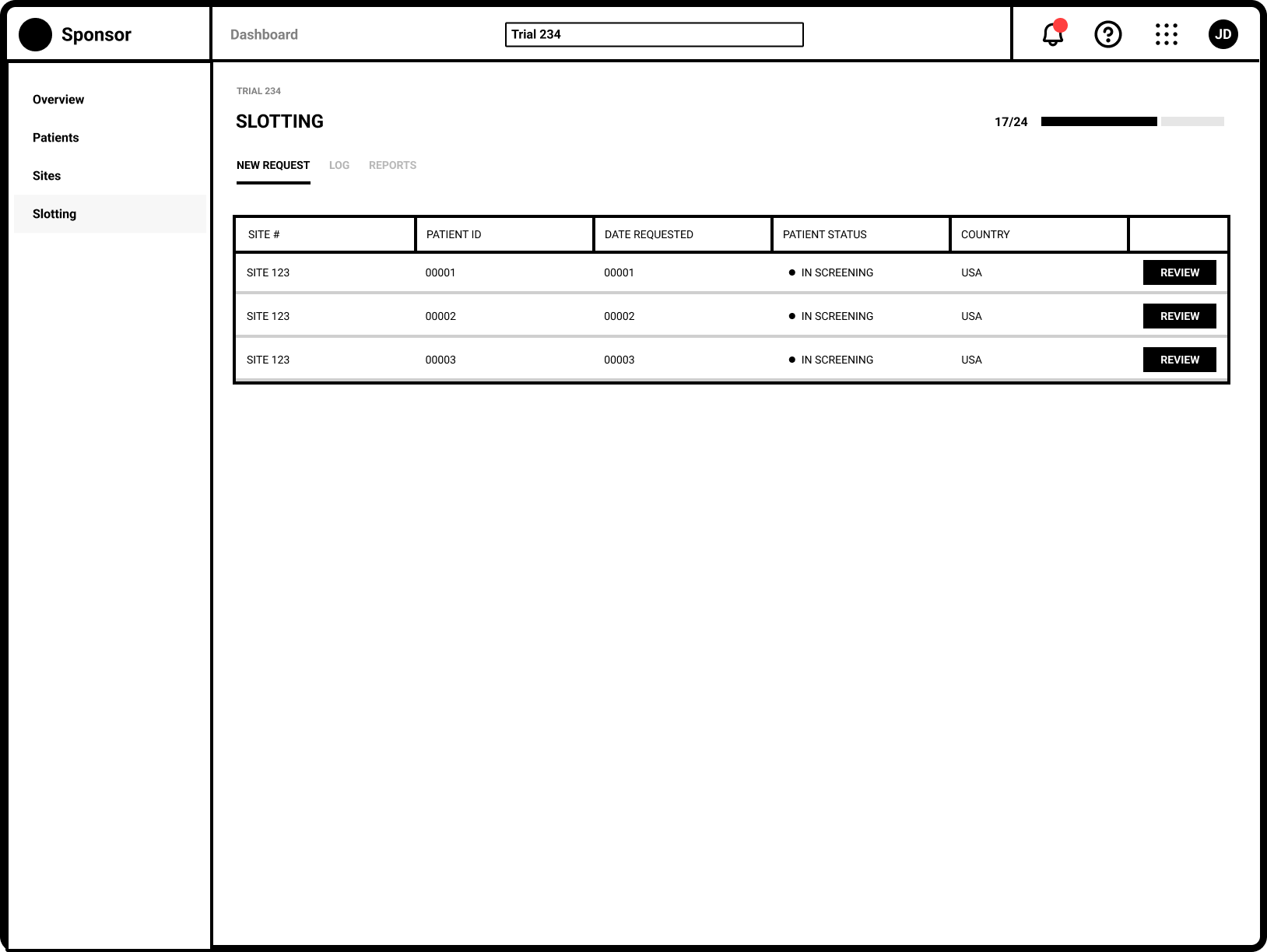
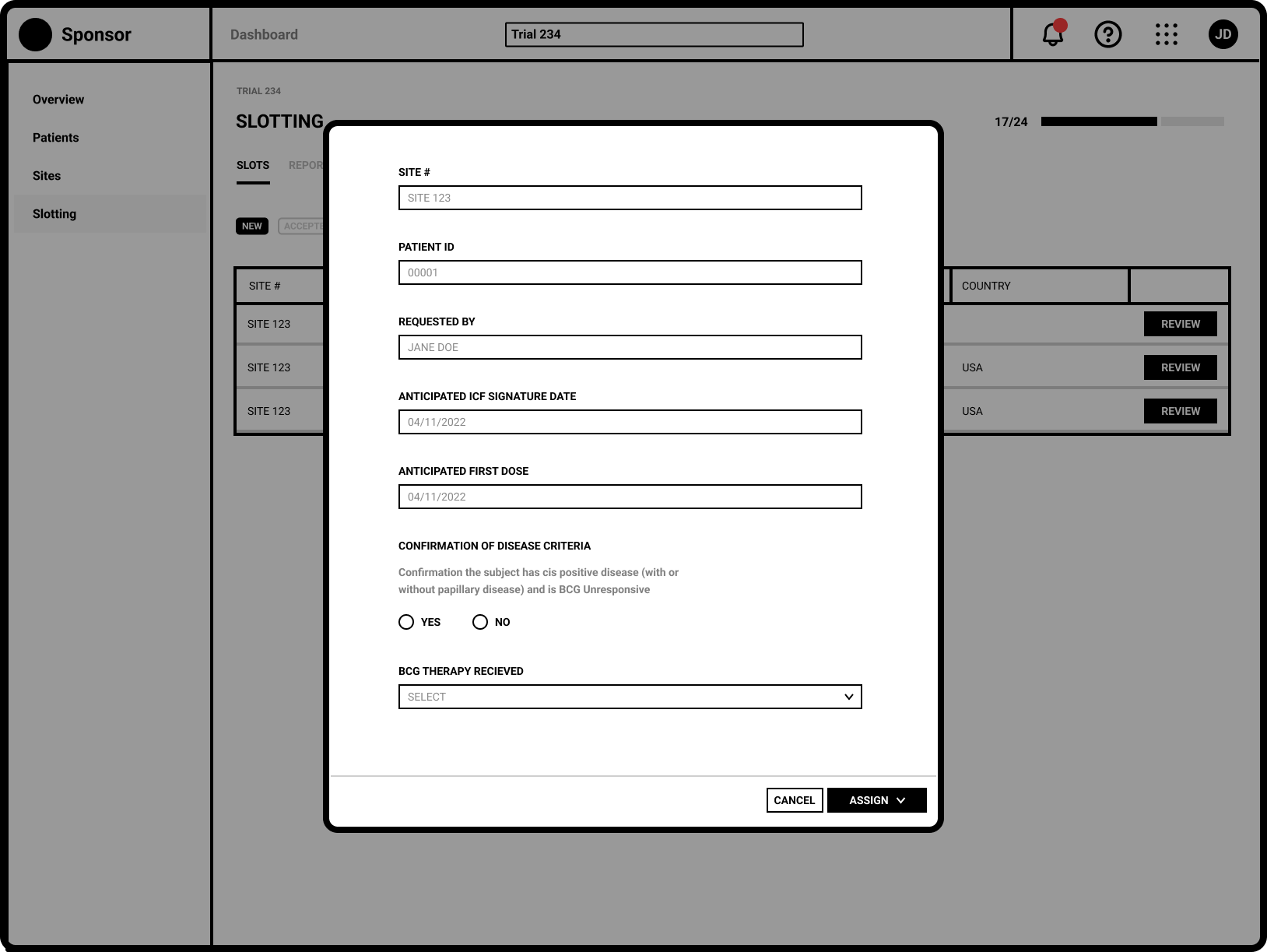
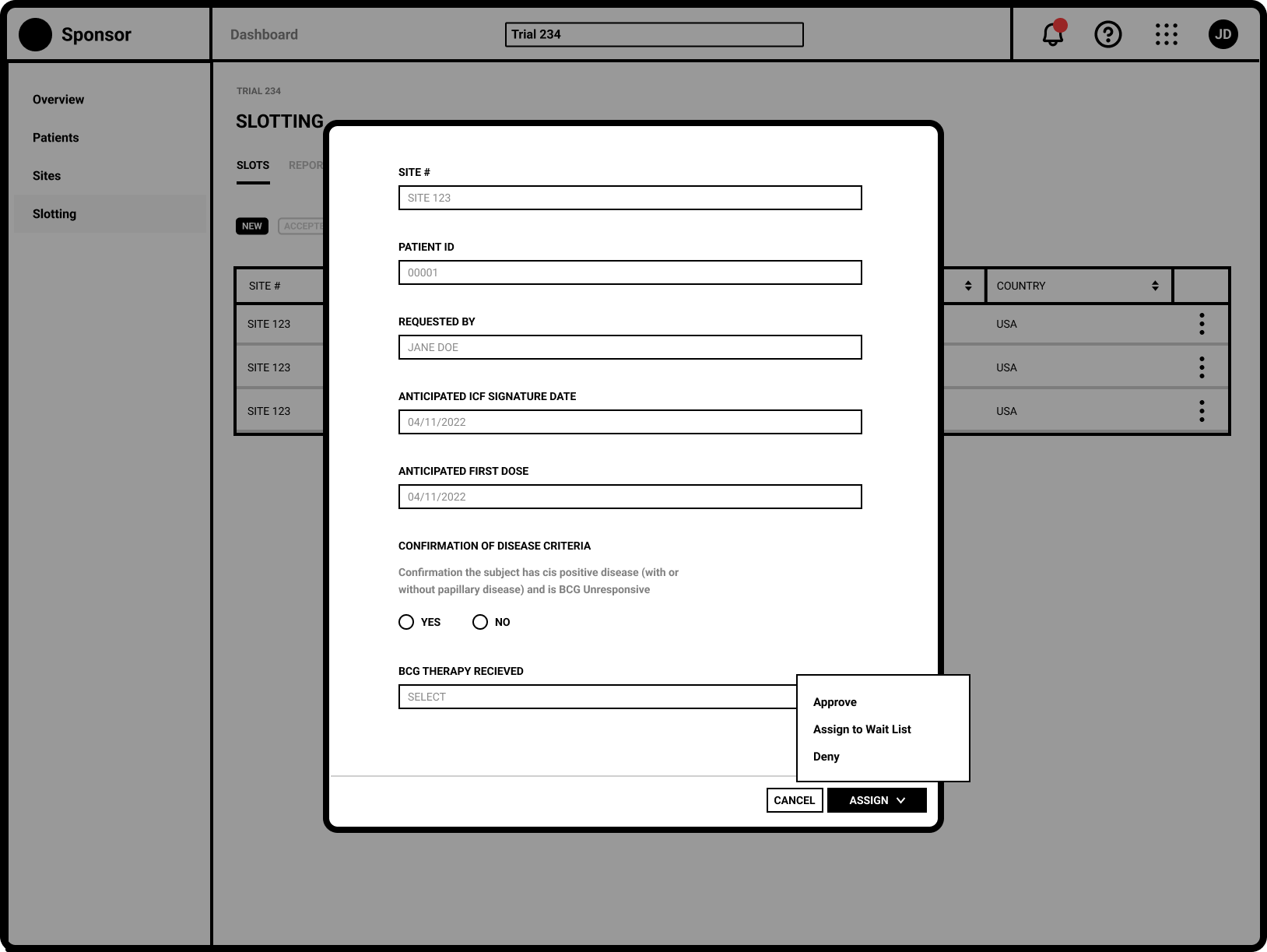
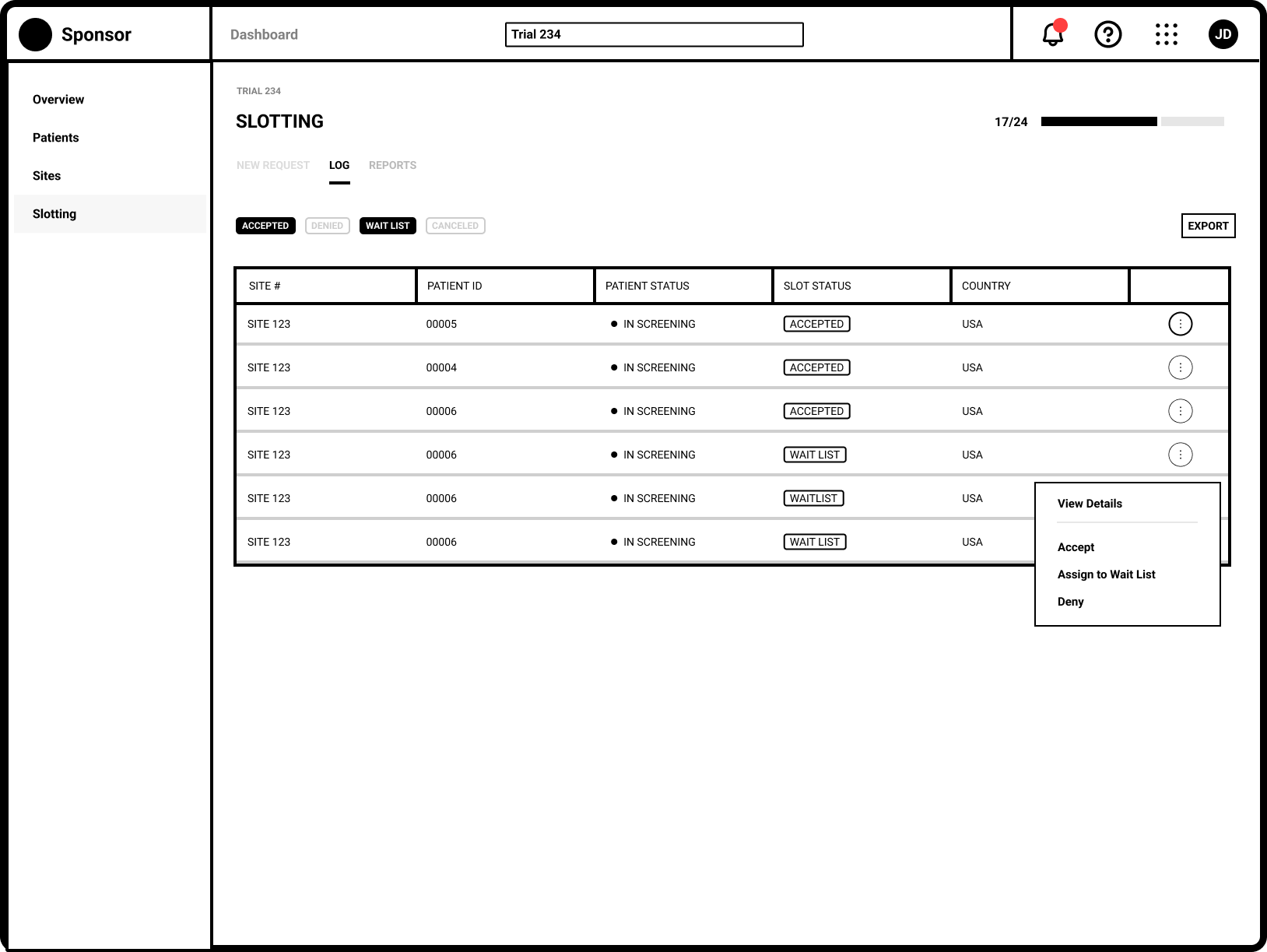
Refinement
Testing with Users
We planned to release early and often. We designed our test plan to accommodate three separate test phases to allow internal stakeholders and a select group of early adopters to test the Slot Management interface within the production environment. The Goal was to review the value proposition and validate functionality incrementally.
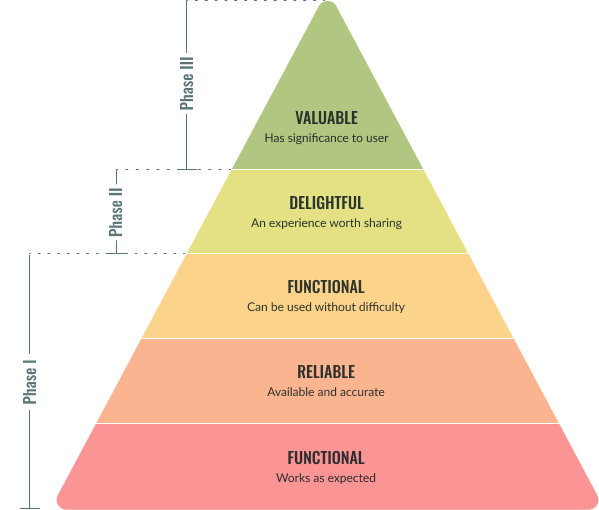
| Phase III (MVP Release) |
|
| Phase II |
|
| Phase I |
|
Key Takeaways
We conducted five 45 minute sessions with individual participants. Particpants were asked perform a series of tasks during each of the sessions. During this time, participants were also asked to provide quantititive ratings to gauge ease of use and overall satisfaction.
Importance of In-App Notifications
95% of users noted the importance of in-app notifications showing where slot requests needed review.
“CTMs have so much on their plates. Managing multiple trials can be difficult. Emails could go to spam. I wouldn't rely solely on email.”
Limited Discoverability of "Request" Action
Several users noted that they would expect the “request” functionality to appear outside of the patient log tab of the patient card.
“I expected it to be really clear but after a bunch of poking around I gave up”
Low Confidence in Cross-App Communication
45% of users were not confident that changes made throughout the lifecycle of a slot request were successfully communicated to the other application.
“I don't know what information I submitted. Did I just submit a request?”
Raising the Fidelity
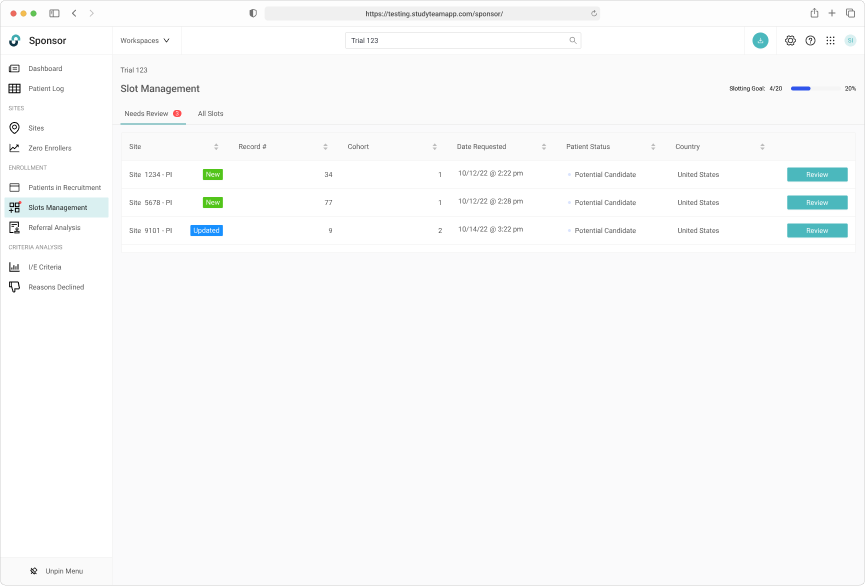
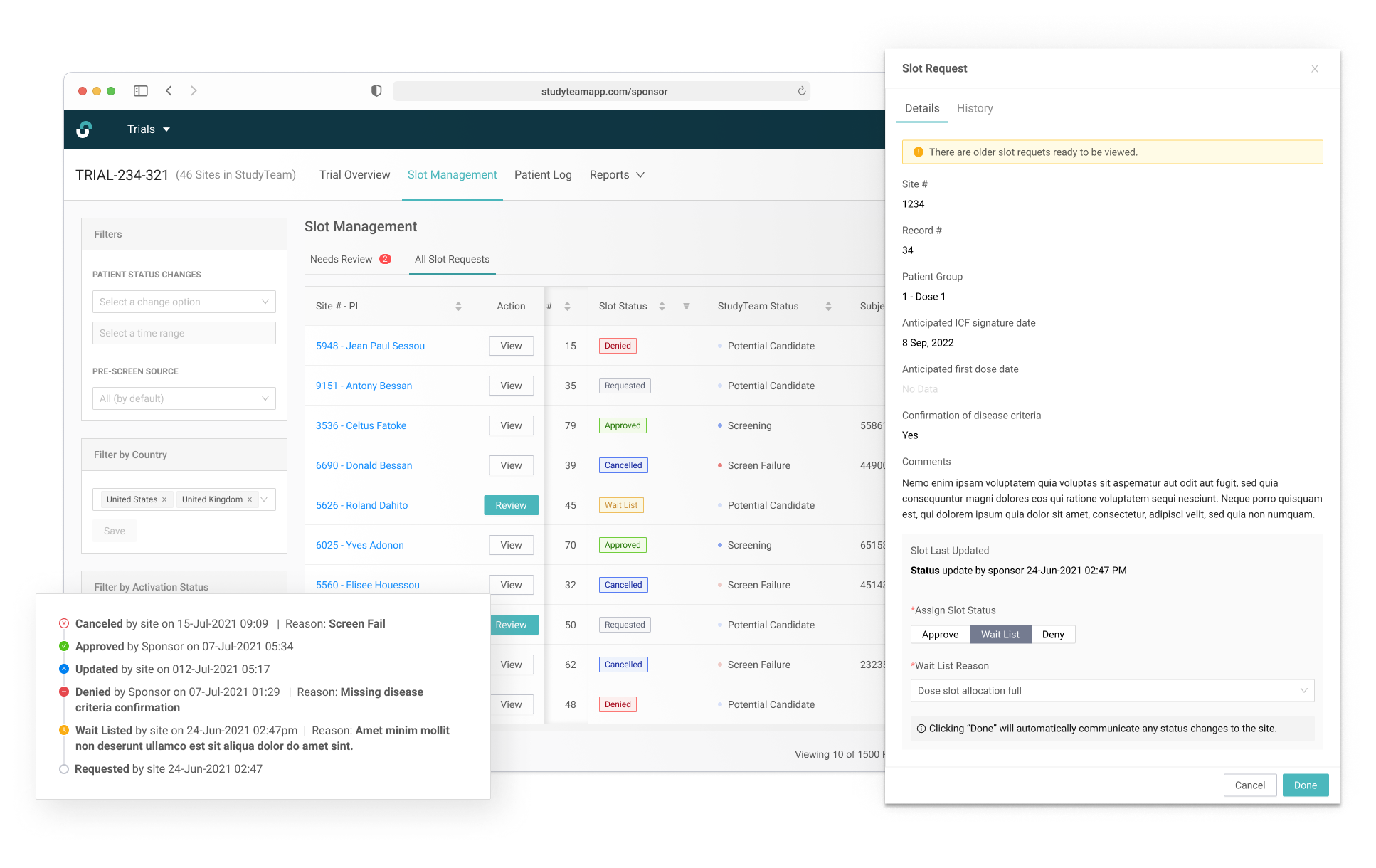
Separating the New and Updated requests from site users allows Trial Managers to focus on request requiring their immediate attention.
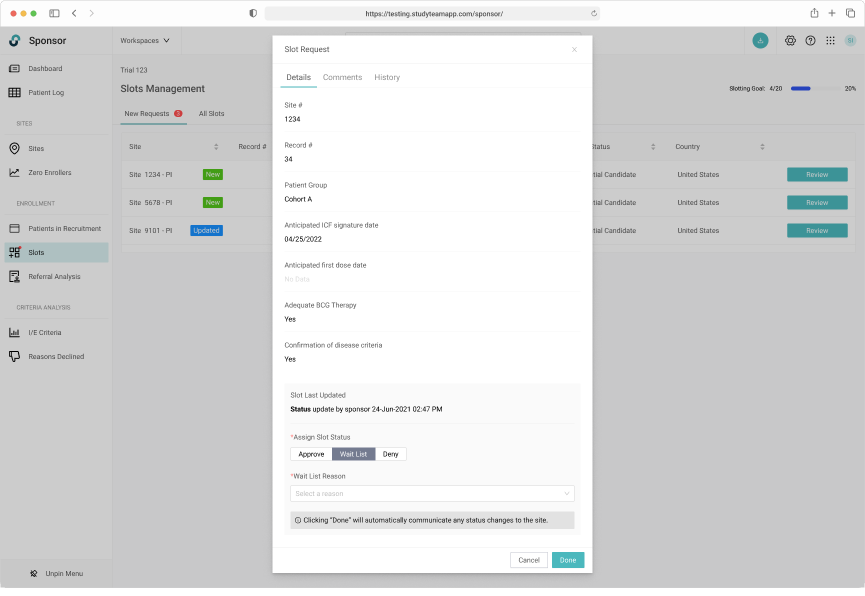
The request specific view shows the Sponsor user all necessary eligibiity criteria.
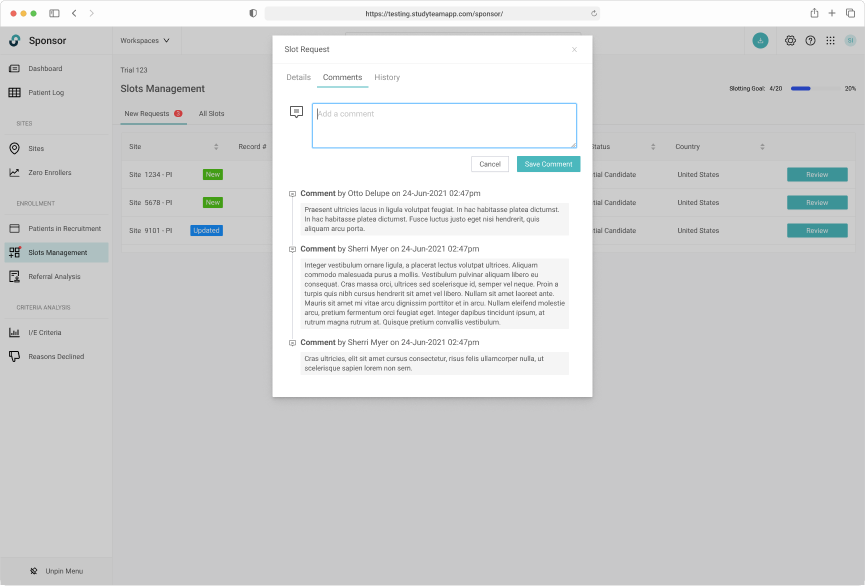
Global trials often have regional and country specific trial managers. A commenting system allows for user communication across multiple regions.
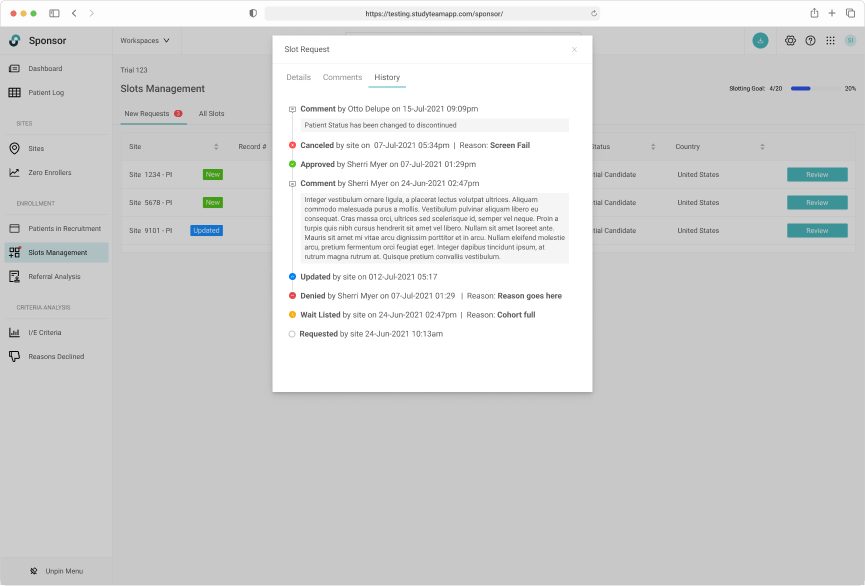
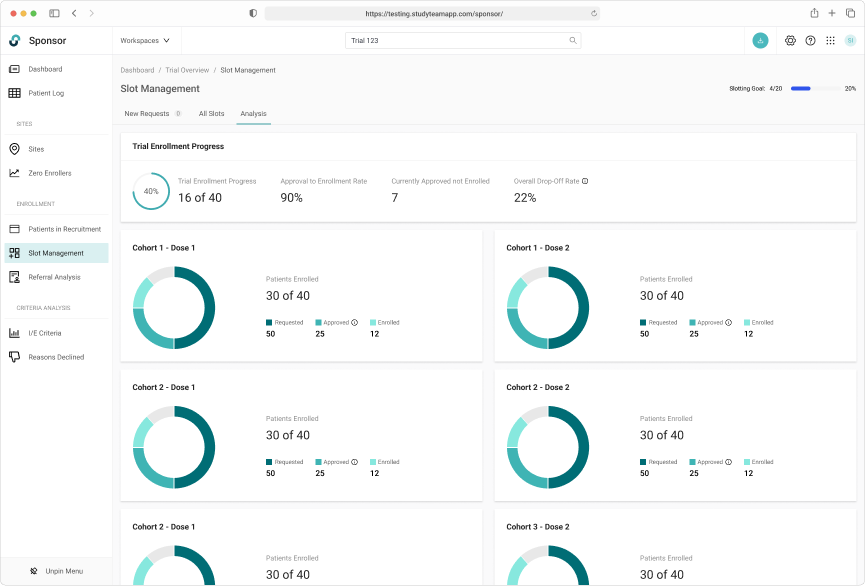
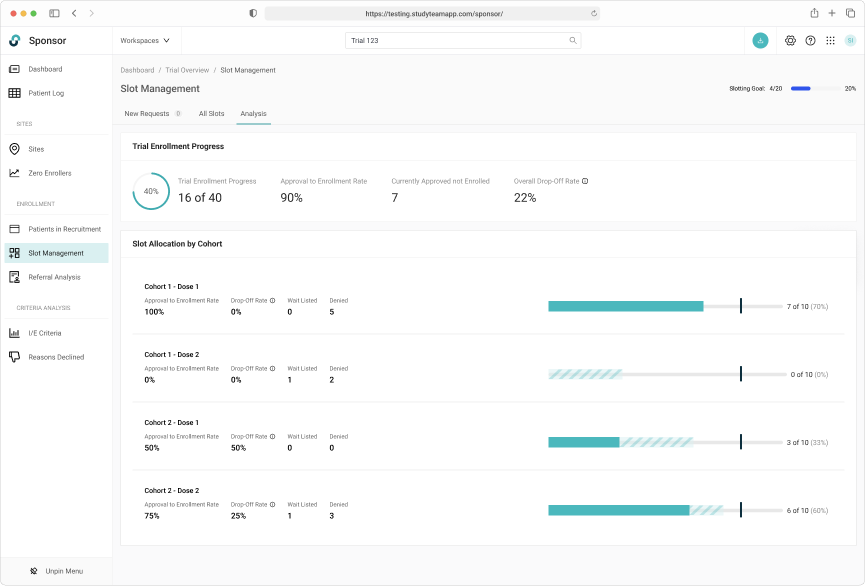
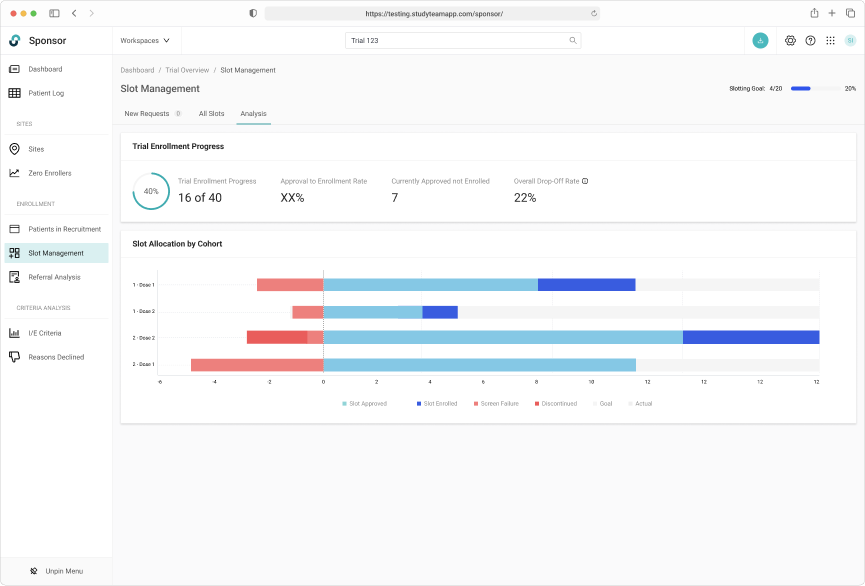
Capturing a complete history of patient allocation for the duration of the trial.
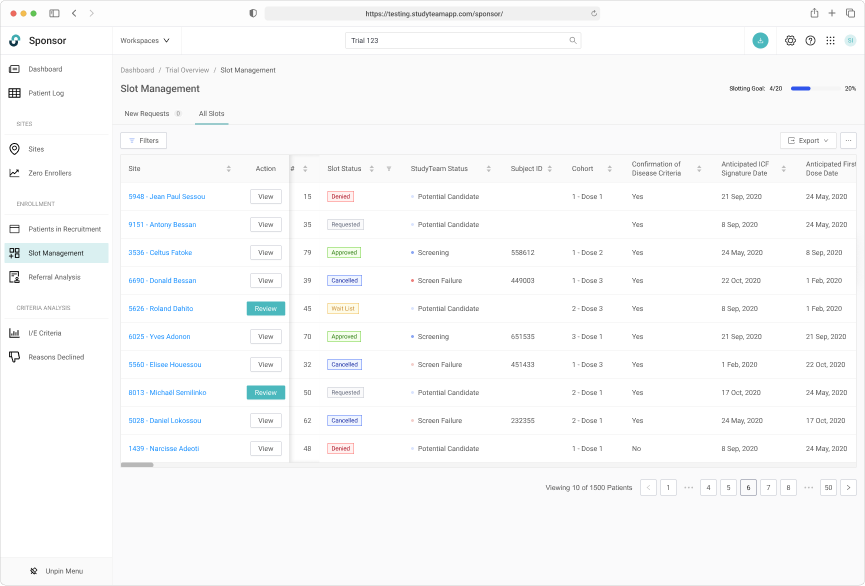
Tracking slot allocate throughout the entire trial lifecycle was key
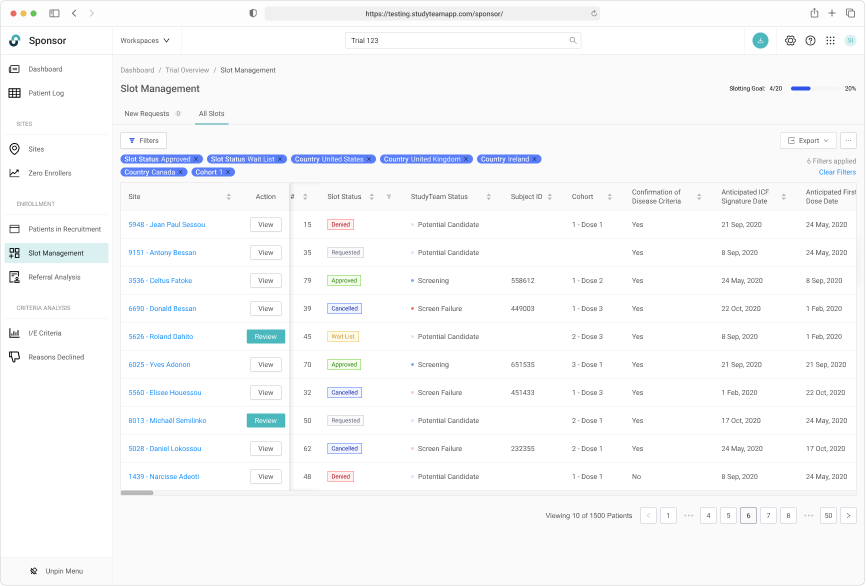
Robust filtering allowed users to easily manage patients on the wait list.
Looking to the Future
Increasing Product Value
Revisiting the company's mission to remove clinical trial operations as the bottleneck on delivering new therapies to patients, brings us to the long-term vision of slot management. Helping sponsors better understand the their enrollment funnel by providing real-time reporting could allow them to better evaluate the necessity of protocol amendments.