Clinical Trial Dashboard
Creating a unified experience to glean insights across multiple clinical trials within a single view.
Setting the Scene
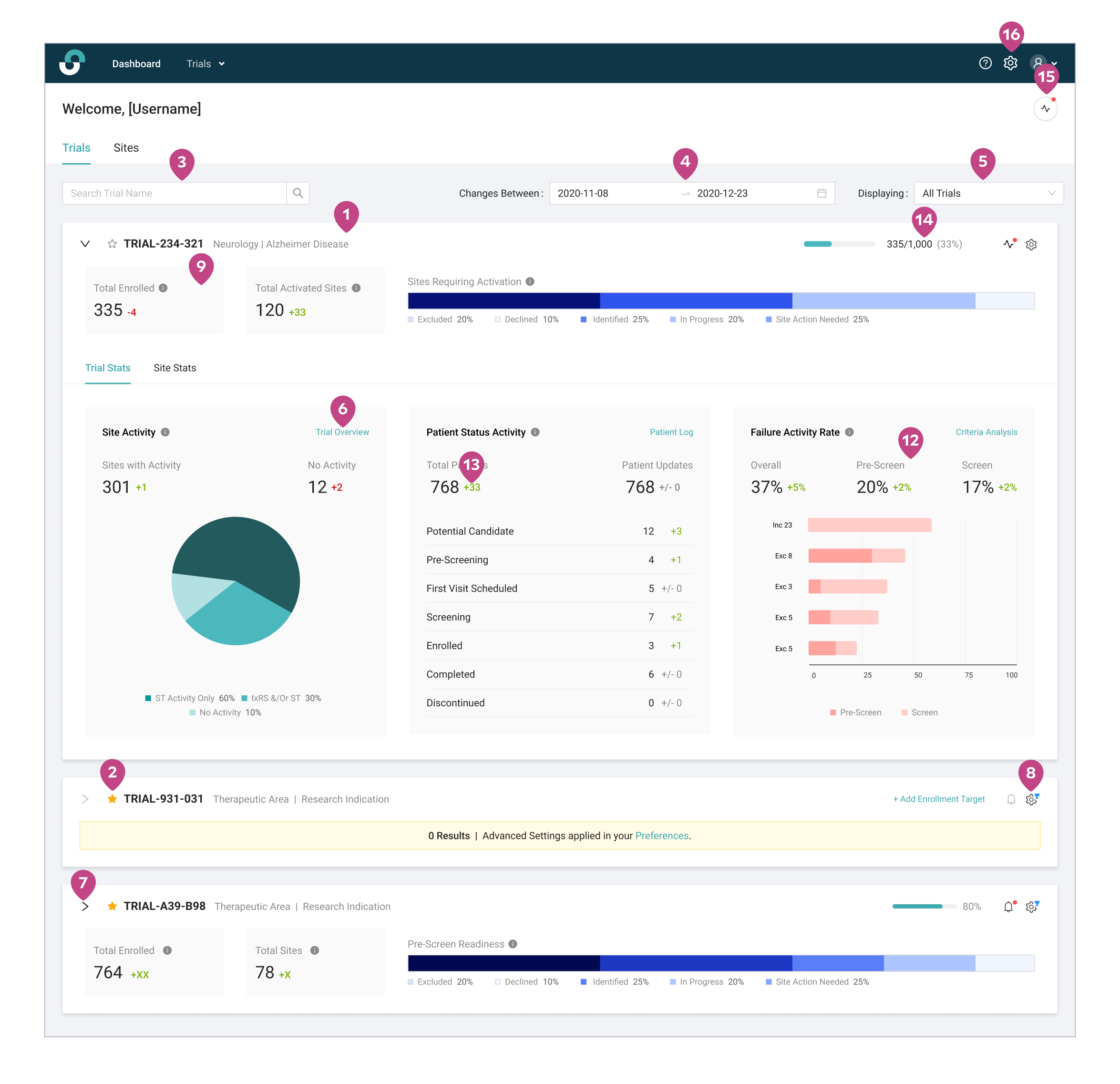
Enrollment Control Center
Onestudyteam (OST) offers cloud-based software solutions to both clinical research sites (Sites), research centers and global biopharmaceutical companies (Sponsors) to accelerate clinical trial enrollment. The company's mission is to remove clinical trial operations as the bottleneck on delivering new therapies to patients. The software aims to harmonize workflows for sites & sponsors.
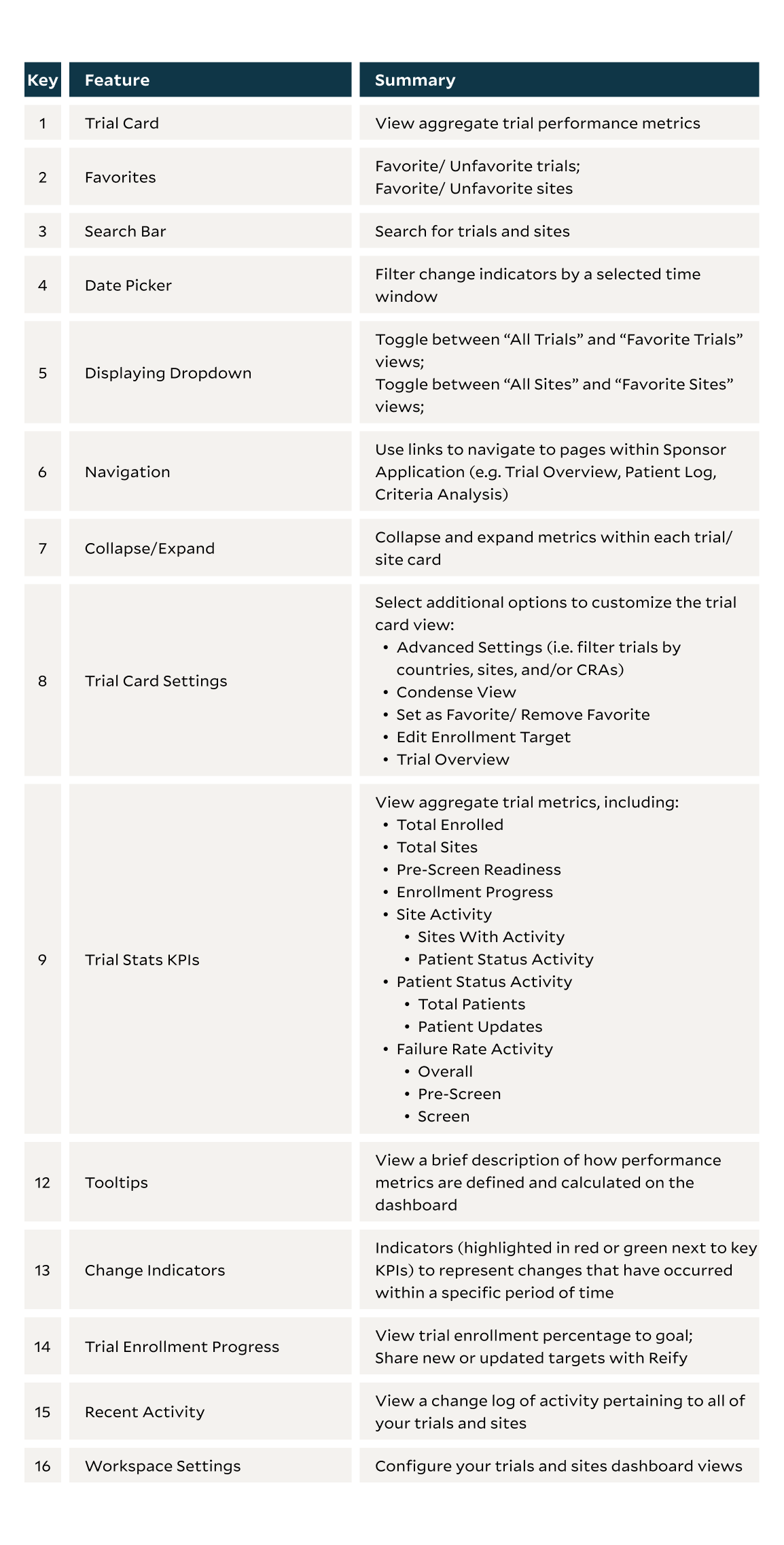
This project explores the addition of a cross-trial view within the OST Sponsor application. We set out to allow sponsor users a configurable, role-based user interface that displayed insights across multiple clinical trials and research sites within a single view. We aimed to enable users to view their trials and sites in one place, and allow them to gain insight to overall trial and site performance.

Why are we here?
Understanding the Problem
The OST sponsor app offered a disjointed user experience without providing any insights across trials or sites. Upon logging in, sponsors were presented with a list of trials and only have the option to view study performance for one trial at a time. Additionally, while there are a number of different sponsor user personas (i.e. CRAs, Trial Managers), there is only one "version" of the Sponsor app available. Depending on the sponsor's role, they may only regularly work with a subset of trials and/or sites. Therefore, in order to make the app as streamlined and user friendly as possible, it was important for sponsors to have the ability to customize their workspace based on their specific needs.
Concise View
A complete picture, across BOTH central recruitment and site-driven recruitment channels so they can draw accurate insights.Site Uptake
An earlier visibility in to recruitment progress and site activity, so we can course-correct before it is too lateEnrollment Dropoff
Better insights on the root cause(s) of enrollment issues, so we can choose the most impactful actions to keep enrollment on plan.Personas
Identifying our Users
The primary users for this project are employees at biopharma companies across two primary job functions. When considering individual user needs based on role, we can break it down a little further.

“I need information at a glance so that I can easily identify successes and problem areas. It is important for me to have the ability to drill down into problem areas so that I can try to fix them.”
Global Trial Manager
Bio
Sponsor manager is responsible for the oversight of recruitment and enrollment across an entire trial at the global level. This person may be our primary point of contact, and is likely reviewing the Enrollment Report/StudyTeam data on a weekly basis (if not more frequently). If protocol amendments are needed, this person is likely the decision maker. The persona responsible for the overall success of the trial.
The CRA is generally responsible for a subset of sites on the trial. Also commonly called CDC or Site Monitor. These are users who we expect to review site-specific Patient Logs in StudyTeam on a regular basis to ensure that their sites are recruiting and if they are not, they will be able to have that conversation with their sites during a future touchpoint.
Education
Bachelor or Masters degree in a Life Science
(biology, microbiology, pharmacology, molecular biology, toxicology or immunology)
Traits
- Excellent computer skills
- Excellent communication and organizational skills
- Knowledge of good clinical practice (GCP)
Goals
- See overall recruitment status for my region (behind, on-target, ahead)
- Identify high incidents of failure for criteria so that I can modify protocol if needed
- See contact information for necessary Sponsor staff when I have questions
- Identify successful/active and unsuccessful/inactive sites during enrollment
- Identify successful and unsuccessful recruitment vendors

Photo by Christina @ wocintechchat.com on Unsplash
“I need to have my data view limited to sites I am responsible for. Sometimes a birds-eye view of the trial isn't helpful when I am trying to monitor and help sites with their recruitment efforts.”
Clinical Research Associate (CRA)
Bio
A Clinical Research Associate (CRA) is responsible for monitoring the study at the site level to ensure compliance with the protocol, regulations, and Good Clinical Practice (GCP) guidelines. They also assist with site selection, initiation, and close-out activities. They may work directly with the sponsor company of a clinical trial, as an independent freelancer or for a Contract Research Organization (CRO). A Clinical Research Associate ensures compliance with the clinical trial protocol, checks clinical site activities, makes on-site visits, reviews Case Report Forms (CRF) and communicates with clinical research investigators.
The CRA is generally responsible for a subset of sites on the trial. Also commonly called CDC or Site Monitor. These are users who we expect to review site-specific Patient Logs in StudyTeam on a regular basis to ensure that their sites are recruiting and if they are not, they will be able to have that conversation with their sites during a future touchpoint.
Education
Bachelor or Masters degree in a Life Science
(biology, microbiology, pharmacology, molecular biology, toxicology or immunology)
Traits
- Excellent computer skills
- Excellent communication and organizational skills
- High level of integrity
- Knowledge of good clinical practice (GCP)
Goals
- See my activated site activity
- See a comprehensive list of all trials at the site for a given sponsor
- Easily navigate across trials at a site
- Easily identify slow recruitment or inactivity at my sites
Jammin' in Lo-fi
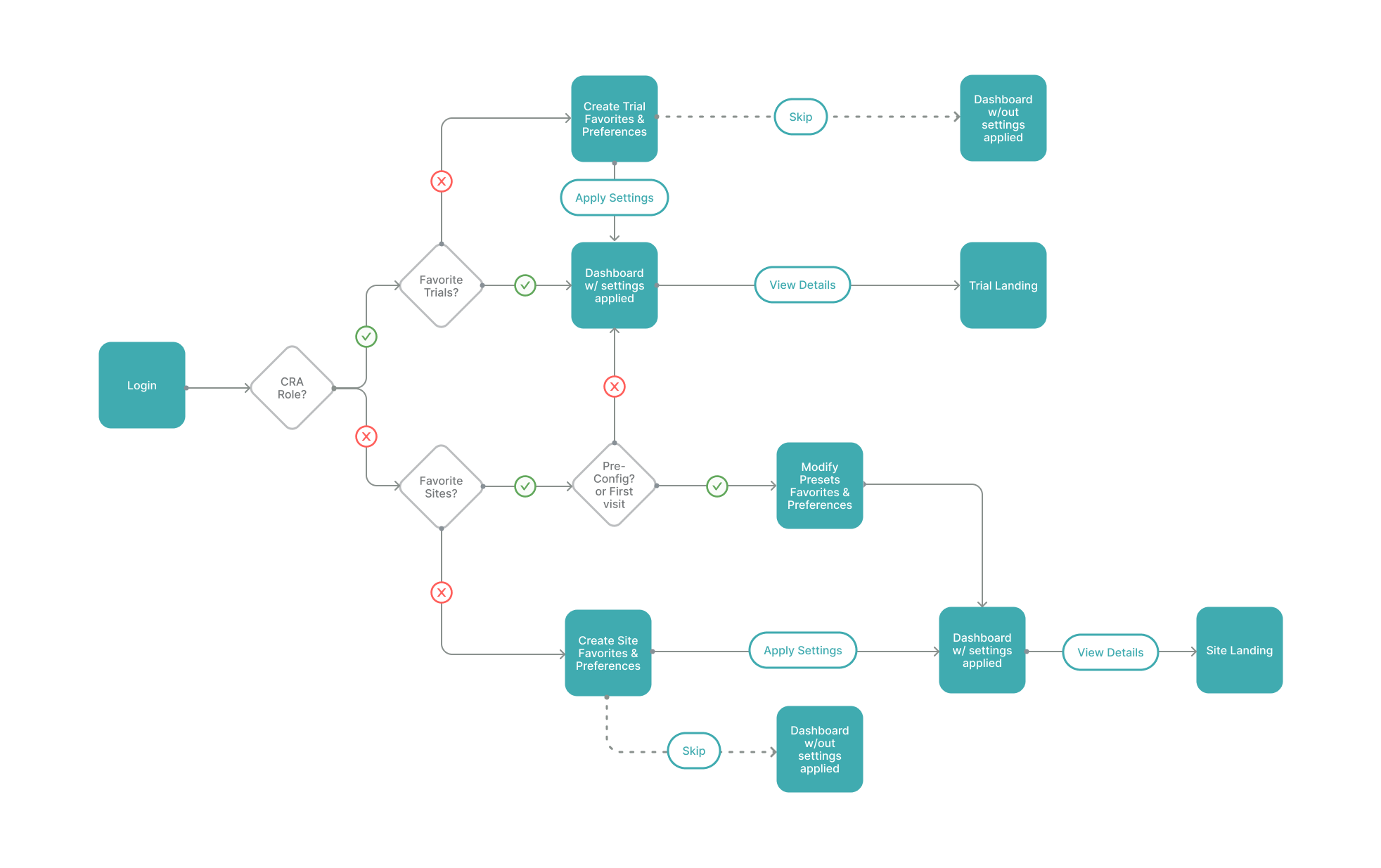
Ideating a Role-Based User Flow
After identifying the two primary personas, product managers and designers partnered to create an onboarding flow. Our goal was to walk users through a meaningful setup process that allowed them to personalize their dashboard experience.
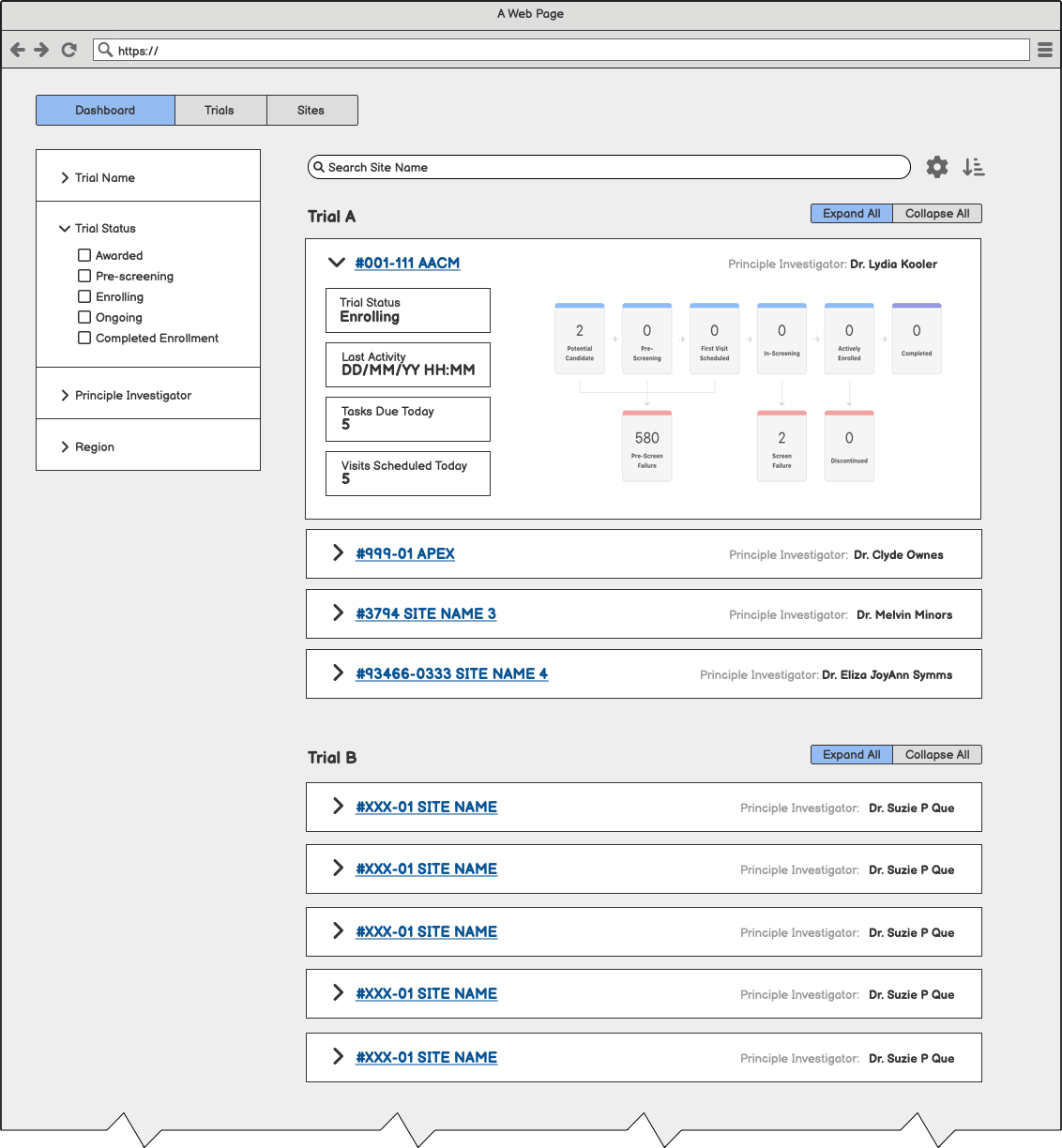
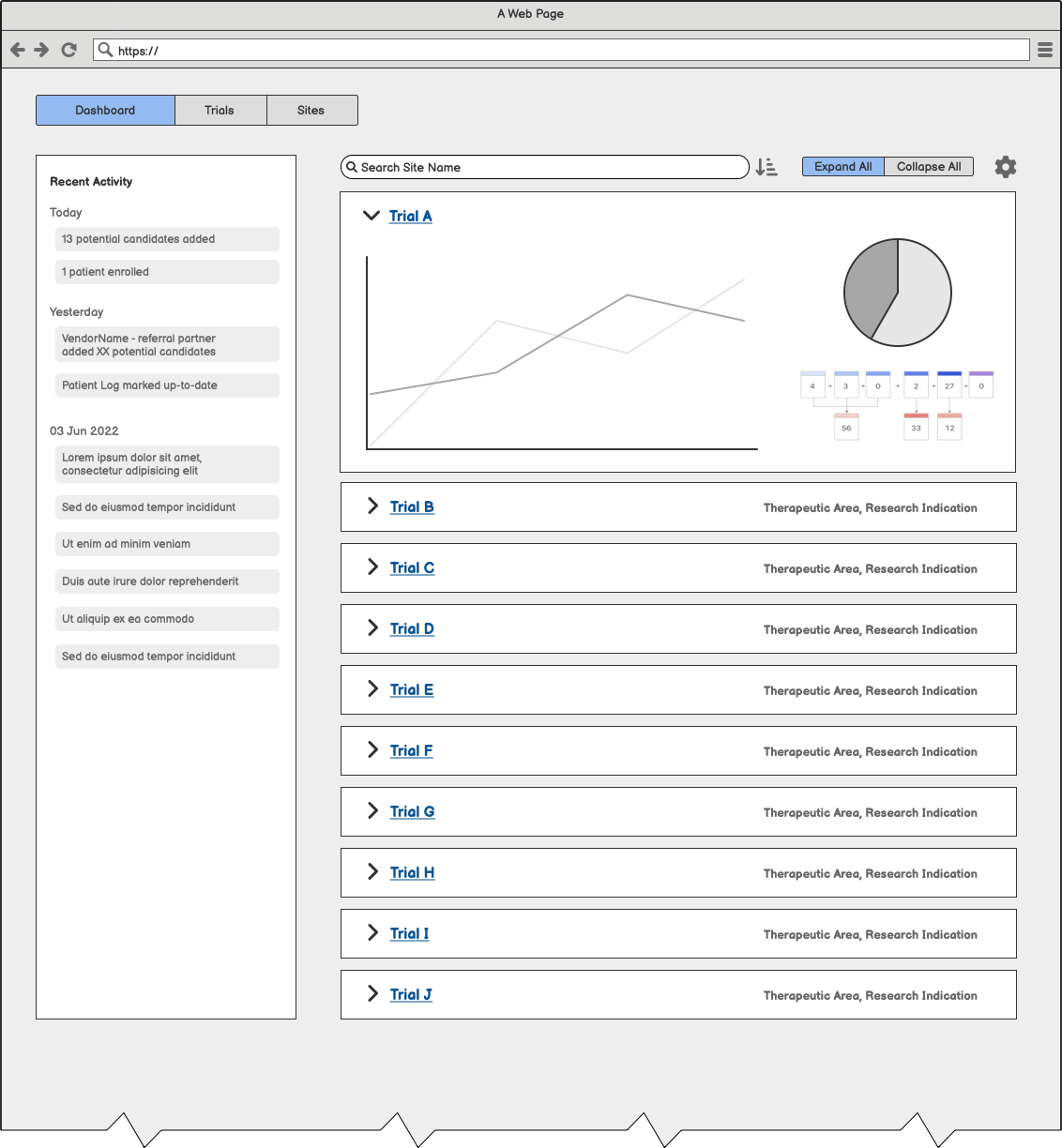
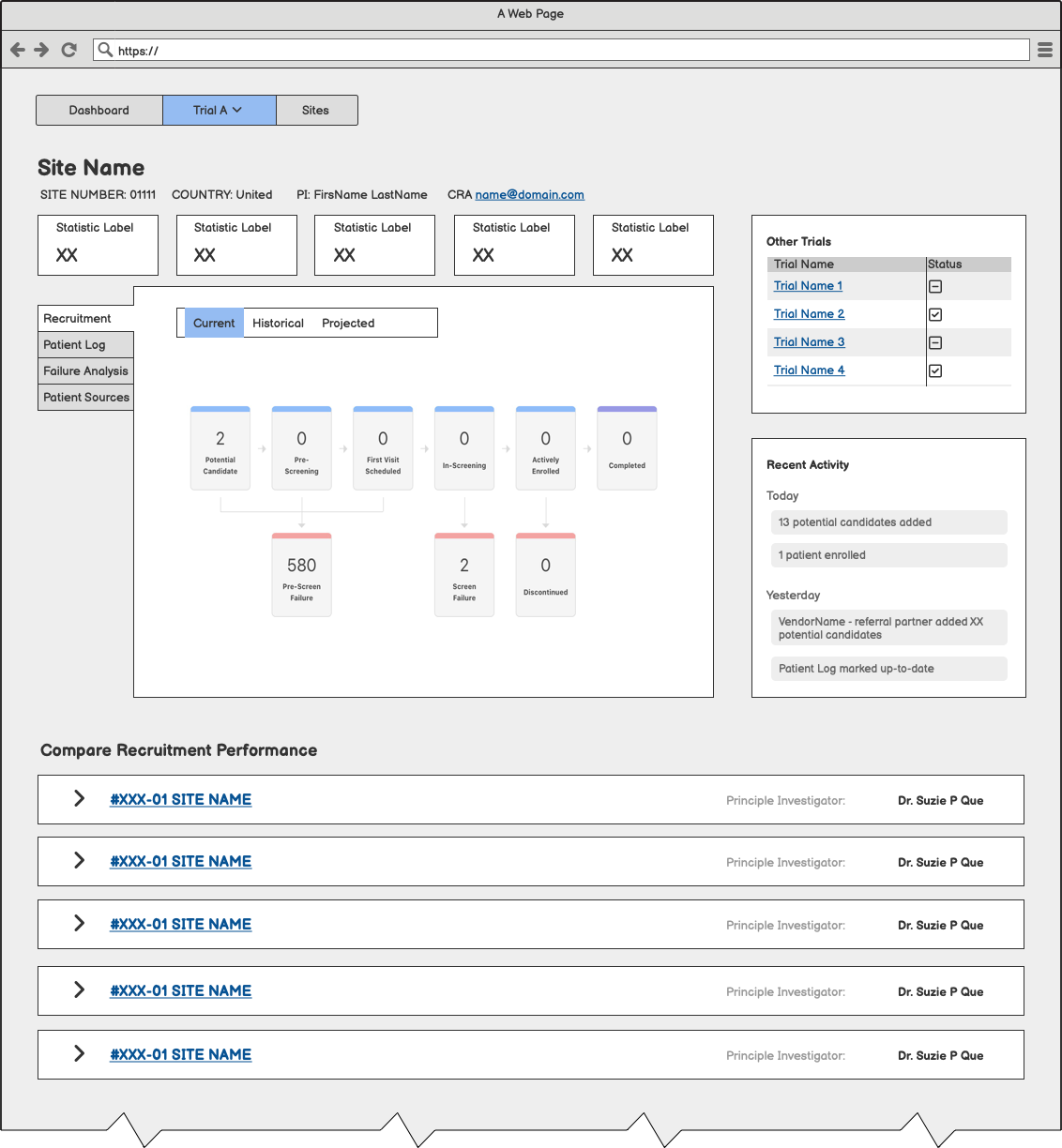
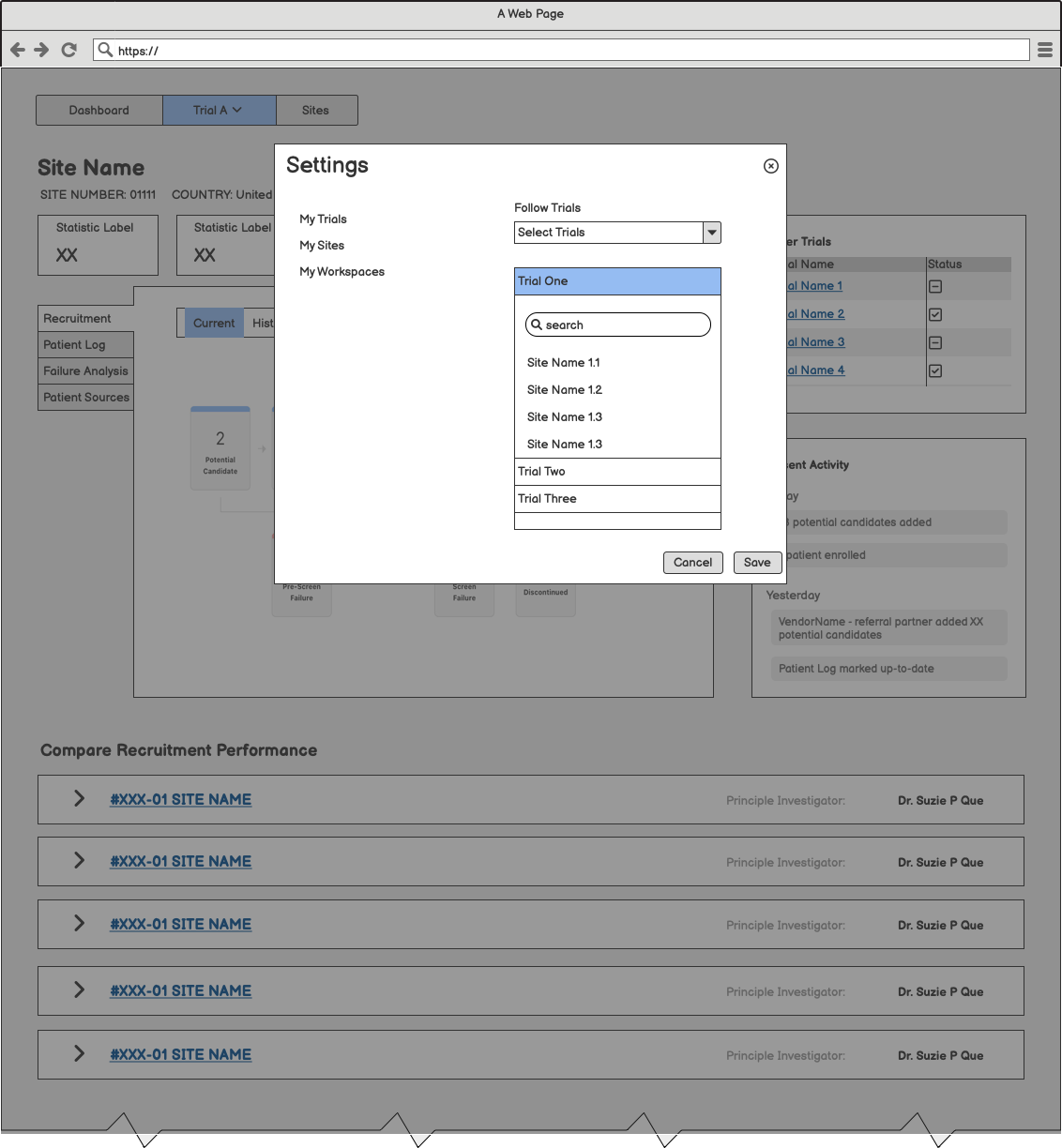
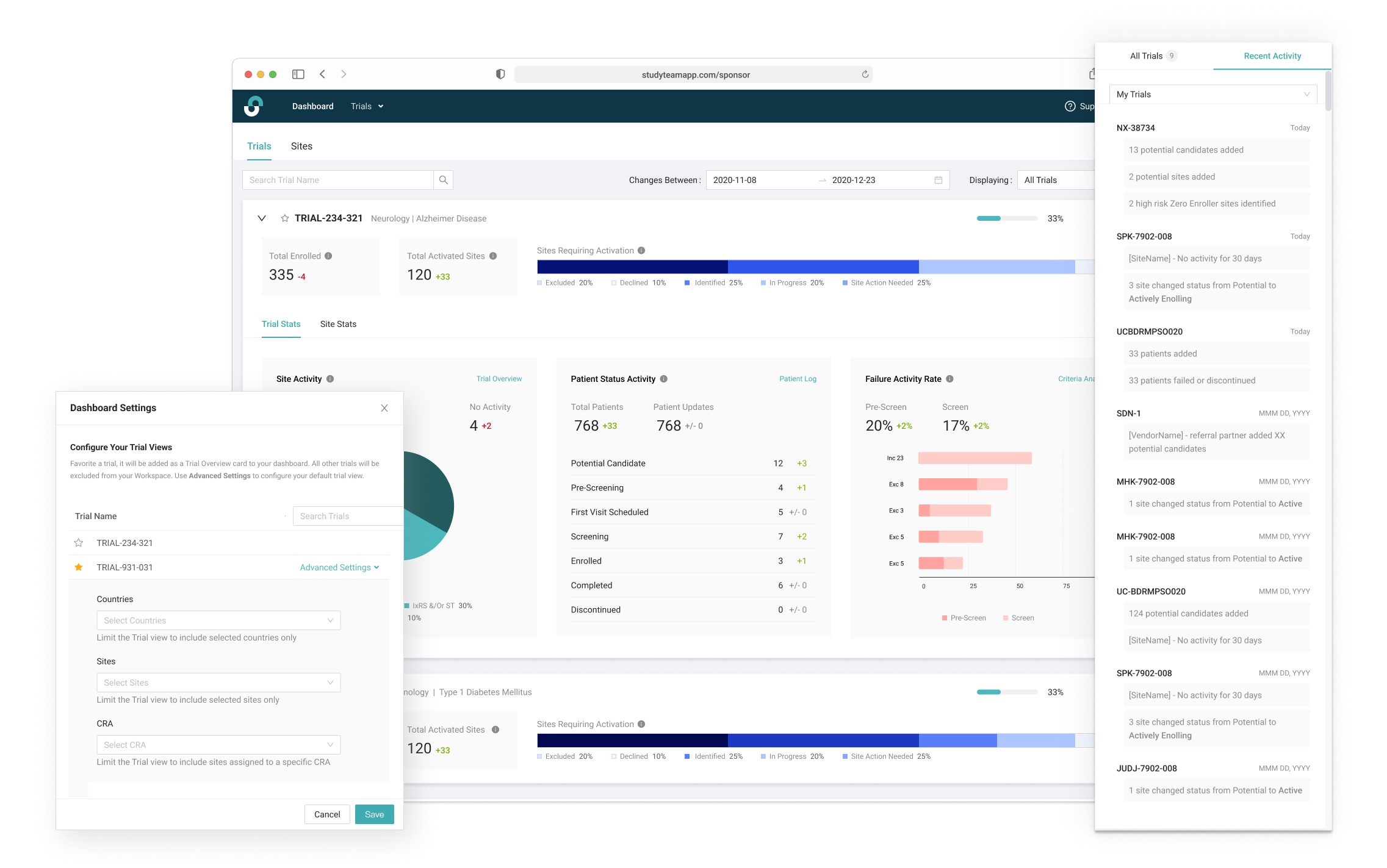
Raising the Fidelity
Choosing Meaningful Metrics
After identifying some possible key metrics for the Trial Manager, we tested three versions of the trial statistics. Our goal was to acertain what level of detail the Global Trial Manager would find most useful. We also aimed to test our hypothesis of presenting high level metrics along with the ability to access more details via other reporting features in the application.
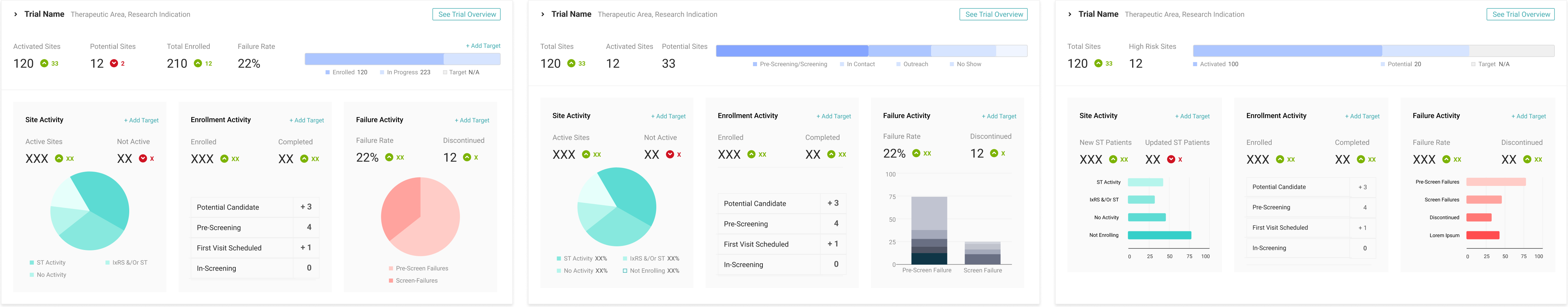
Three layouts provided for initial prototype feedback
Testing
Alpha Release
We conducted a somewhat large scale alpha test prior to release. Why not start small, you ask. Sometimes due to development contraints and unforeseen slow downs, we need to pivot. The initial testing timeframe was schedule for approximately 4 weeks. During that time we hoped to identify usability issues, bugs and gather general qualitative feedback. As we all know, sometimes things do not go according to plan. As our launch date quickly approached, we re-organized our test plan to include a large-scale internal alpha test that included 49 participants.
After providing each participant with a testing script that included a series of tasks, we asked them to complete a survey rating their experience. Our survey used Likert scales assess the degree to which a participant agreed or disagreed with a given statement. For example, participants were asked to use a 1-5 scale where 1 represented “I strongly disagree” and 5 represented “I strongly agree” to communicate how strongly feel about a particular statement.
Overall, the qualitative feedback boiled down to the sentiment that the dashboard was aesthetically pleasing and gave visual delight, but the amount of data was somewhat overwhelming. Moreover, the dashboard felt sluggish and non-performant.
57.1%
Agreed that Sponsor users would be likely to utilize and customized the dashboard.
“I think they are really going to like it!”
81.7%
Did not feel confident the feature was ready for launch. Nearly 50% of particpants cited load times and performance issues in followup qualitative statements.
“It takes a while for everything to load, but that was my only issue.”
65.4%
Customer success members did not feel confident supporting the feature launch.
“I think that we need to improve the loading times before launching.”
How did we do?
Measuring Success
Particpant tracking was used to monitor application usage of 667 individuals. The individuals identified were users who responded to a qualitative survey and used the dashboard for the first 30 days after the public release.
Using login data and poll responses from the individuals, it appeared that the Sponsor Dashboard had positively impacted user logins. When compareding login activity, we saw that 69% of users had increased usage of the sponsor applicaton.

of early adoptors agreed the dashboard improved their experience
"Working across multiple trials and therapeutic areas, it is fantastic to be able to login to one dashboard that shows all of my most up-to-date enrollment and SR information across the projects I work. It's visually well-designed to make it super easy to get a macro update at a glance."
"I think it is very well designed visually. Rarely do healthcare tech platforms have the look and ease of use that recreational tech platforms have. This software and now the sponsor platform feels like it was designed by Apple or Google which is the highest praise this 30-something nurse has to offer."